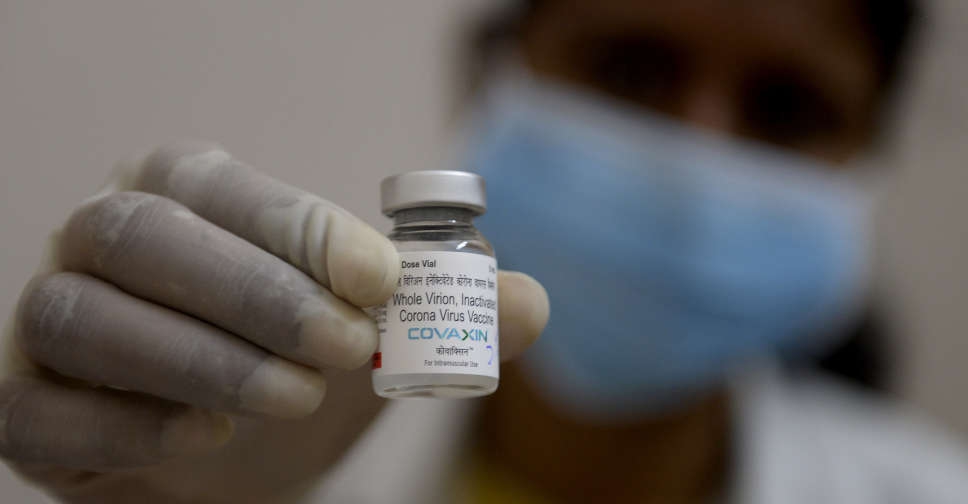
A World Health Organisation (WHO) technical advisory group was reviewing data on India's Covaxin shot against COVID-19 on Tuesday with a decision on its emergency use listing likely soon, a spokesperson said.
"If all is in place and all goes well and if the committee is satisfied, we would expect a recommendation within the next 24 hours or so," Margaret Harris told journalists at a UN press briefing.
Millions of Indians have taken the shot produced by Bharat Biotech but many have been unable to travel pending the WHO approval.




 Israeli strikes kill 26 in Gaza, health officials say
Israeli strikes kill 26 in Gaza, health officials say
 US government starts likely brief shutdown as House fails to approve deal
US government starts likely brief shutdown as House fails to approve deal
 Thousands demonstrate in Minnesota and across US to protest ICE
Thousands demonstrate in Minnesota and across US to protest ICE
 France tightens infant milk rules after recalls
France tightens infant milk rules after recalls



